- Number Of Electrons In A Silicon Atom
- Iron Element Configuration
- Number Of Electrons In Silicon Atom
- Number Of Electrons In Silicon In Outer Shell
- Number Of Free Electrons In Silicon
- Assertion: The number of electrons in a p-type silicon semiconductor is less than the number of electrons in a pure silicon semiconductor at room temperature. Reason: It is due to law of mass action.
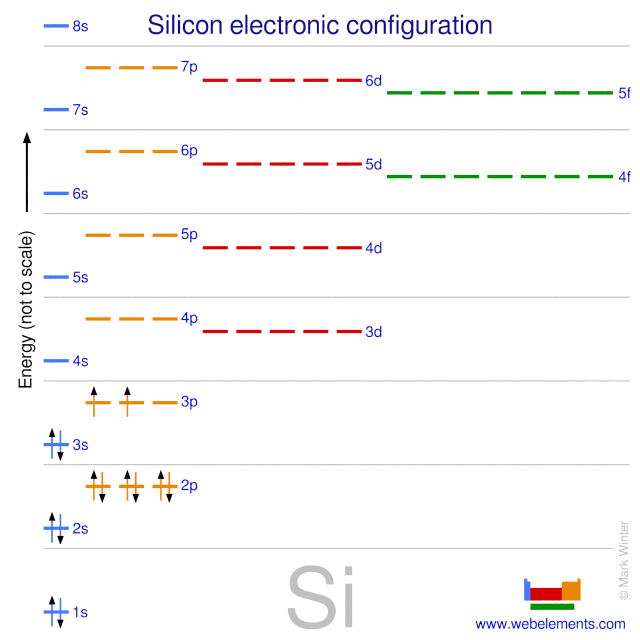
- In order to write the Silicon electron configuration we first need to know the number of electrons for the Si atom (there are 14 electrons). When we write the configuration we'll put all 14 electrons in orbitals around the nucleus of the Silicon atom.
Charge carrier density, also known as carrier concentration, denotes the number of charge carriers in per volume. In SI units, it is measured in m−3. As with any density, in principle it can depend on position. However, usually carrier concentration is given as a single number, and represents the average carrier density over the whole material.
Number Of Electrons In A Silicon Atom
Charge carrier densities involve equations concerning the electrical conductivity and related phenomena like the thermal conductivity.
Calculation[edit]
The carrier density is usually obtained theoretically by integrating the density of states over the energy range of charge carriers in the material (e.g. integrating over the conduction band for electrons, integrating over the valence band for holes).
If the total number of charge carriers is known, the carrier density can be found by simply dividing by the volume. To show this mathematically, charge carrier density is a particle density, so integrating it over a volume gives the number of charge carriers in that volume
.
Iron Element Configuration
where
- is the position-dependent charge carrier density.
If the density does not depend on position and is instead equal to a constant this equation simplifies to
.
The number of electrons in the penultimate shell of silicon 2 See answers seemajain0008 seemajain0008 Answer. There are 14 electrons in a silicon atom.
Semiconductors[edit]
The carrier density is important for semiconductors, where it is an important quantity for the process of chemical doping. Using band theory, the electron density, is number of electrons per unit volume in the conduction band. For holes, is the number of holes per unit volume in the valence band. To calculate this number for electrons, we start with the idea that the total density of conduction-band electrons, , is just adding up the conduction electron density across the different energies in the band, from the bottom of the band to the top of the band .
Because electrons are fermions, the density of conduction electrons at any particular energy, is the product of the density of states, or how many conducting states are possible, with the Fermi–Dirac distribution, which tells us the portion of those states which will actually have electrons in ″them″
In order to simplify the calculation, instead of treating the electrons as fermions, according to the Fermi–Dirac distribution, we instead treat them as a classical non-interacting gas, which is given by the Maxwell–Boltzmann distribution. This approximation has negligible effects when the magnitude , which is true for semiconductors near room temperature. This approximation is invalid at very low temperatures or an extremely small band-gap.
The three-dimensional density of states is:
Number Of Electrons In Silicon Atom
After combination and simplification, these expressions lead to:
Number Of Electrons In Silicon In Outer Shell
A similar expression can be derived for holes. The carrier concentration can be calculated by treating electrons moving back and forth across the bandgap just like the equilibrium of a reversible reaction from chemistry, leading to an electronic mass action law. The mass action law defines a quantity called the intrinsic carrier concentration, which for undoped materials:
The following table lists a few values of the intrinsic carrier concentration for intrinsic semiconductors.
| Material | Carrier density (1/cm³) at 300K |
|---|---|
| Silicon[1] | 9.65×109 |
| Germanium[2] | 2.33×1013 |
| Gallium Arsenide[3] | 2.1×106 |
These carrier concentrations will change if these materials are doped. For example, doping pure silicon with a small amount of phosphorus will increase the carrier density of electrons, n. Then, since n > p, the doped silicon will be a n-type extrinsic semiconductor. Doping pure silicon with a small amount of boron will increase the carrier density of holes, so then p > n, and it will be a p-type extrinsic semiconductor.
Metals[edit]
The carrier density is also applicable to metals, where it can be calculated from the simple Drude model. In this case, the carrier density (in this context, also called the free electron density) can be calculated by:[4]
Where is the Avogadro constant, Z is the number of valence electrons, is the density of the material, and is the atomic mass.
Measurement[edit]
The density of charge carriers can be determined in many cases using the Hall effect,[5] the voltage of which depends inversely on the carrier density.
References[edit]
- ^Pietro P. Altermatt, Andreas Schenk, Frank Geelhaar,Gernot Heiser (2003). 'Reassessment of the intrinsic carrier density in crystalline silicon in view of band-gap narrowing'. Journal of Applied Physics. 93 (3): 1598. doi:10.1063/1.1529297.CS1 maint: multiple names: authors list (link)
- ^O. Madelung, U. Rössler, M. Schulz (2002). 'Germanium (Ge), intrinsic carrier concentration'. Group IV Elements, IV-IV and III-V Compounds. Part b - Electronic, Transport, Optical and Other Properties. Landolt-Börnstein - Group III Condensed Matter. pp. 1–3. doi:10.1007/10832182_503. ISBN978-3-540-42876-3.CS1 maint: multiple names: authors list (link)
- ^Rössler, U. (2002). 'Gallium arsenide (GaAs), intrinsic carrier concentration, electrical and thermal conductivity'. Group IV Elements, IV-IV and III-V Compounds. Part b - Electronic, Transport, Optical and Other Properties. Landolt-Börnstein - Group III Condensed Matter. pp. 1–8. doi:10.1007/10832182_196. ISBN978-3-540-42876-3.
- ^Ashcroft, Mermin. Solid State Physics. p. 4.
- ^Edwin Hall (1879). 'On a New Action of the Magnet on Electric Currents'. American Journal of Mathematics. 2 (3): 287–92. doi:10.2307/2369245. JSTOR2369245. Archived from the original on 27 July 2011. Retrieved 28 February 2008.CS1 maint: discouraged parameter (link)
Number Of Free Electrons In Silicon

